|
Cloud-Based System, Featuring Wearable Biosensor with Chest-Based SpO2, Enables Active Patient Monitoring Across Hospital and Out-of-hospital Care Settings
- Replaces time-consuming manual spot checking with continuous, near real-time active patient monitoring
- Web-based system displays multiple patient physiological data continuously, with visual alarms and alert notifications for streamlined patient care
- Developed to support scalable population health management
MILPITAS, Calif., Nov. 25, 2024 /PRNewswire/ -- LifeSignals, Inc. today announced that the UbiqVue 2A Multiparameter System has received FDA Class II 510(k) clearance, marking a significant milestone in continuous wireless patient monitoring for population health management. UbiqVue 2A Multiparameter System can be deployed in home, remote, and hospital settings – to continuously monitor patients' physiological data, enhancing patient safety and replacing laborious and potentially inaccurate spot-checks.
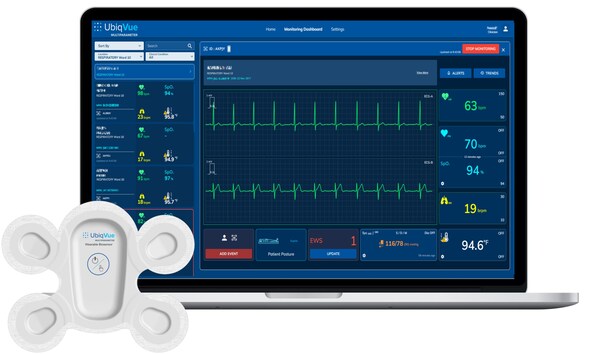
Central to the system is the UbiqVue 2A Biosensor, a single-use, all-in-one wearable device that enables SpO2** to be continuously collected from the chest alongside other biodata for generating a total of twelve other monitored parameters, including 2-channel ECG, pulse rate, PPG, respiration rate, body temperature, and motion. The encrypted data is securely transmitted, in near real-time, from the Biosensor via a relay app or an access point to a secure cloud-based system, where it is further signal-processed. Healthcare professionals and care providers can access continuous vital signs from anywhere, via the UbiqVue web portal, and receive alert notifications. Designed for compatibility with clinical workflows, UbiqVue ensures seamless integration and scalability across healthcare systems.
"This FDA 510(k) approval marks a major milestone in LifeSignals' mission to deliver bedside patient monitor-like functionality through an affordable, single-use disposable Biosensor, enabling population health management," said Surendar Magar, Co-founder and CEO. "It shows our commitment to the vision of expanding the UbiqVue system with additional vital signs and advanced AI capabilities. Through global partnerships with OEMs, service providers, IDTFs, and distributors, we aim to transform healthcare at scale."
"This approval reflects our team's expertise in solving complex engineering challenges and achieving scalable, reliable patient monitoring solutions," said Thomas Varghese, Chief Engineering Officer. "From silicon design and wireless coexistence to collecting multiple vital signs from the chest, the UbiqVue System demonstrates the innovation required to meet the growing demands of modern healthcare."
"The FDA clearance underscores our commitment to delivering scalable solutions," said Saravanan Balasubramanian, VP of Medical Technology & Regulatory Operations. "The UbiqVue System combines the critical parameters of a bedside monitor with a low-cost, single-use Biosensor, enabling streamlined care. We addressed SpO2 accuracy across diverse skin tones, ensured data security, maintained wireless reliability, and met latency requirements for timely alert generation."
UbiqVue is expected to reshape individual patient care and population health strategies, reinforcing LifeSignals' mission to deliver innovative wireless solutions for healthcare systems globally. Learn more: www.lifesignals.com
** White light spectral SpO2 patented technology licensed from BioIntelliSense, Inc. LifeSignals, Inc. in partnership with BioIntelliSense, carried out the product-level design and processing technology enhancements enabling reliable, continuous SpO2 monitoring and accurate performance across all skin tones in a chest-worn Biosensor.